HaCaT Cell
HaCaT Cells: Driving Innovation In Skin And Wound Healing Research
Have you ever had challenges with inconsistent in vitro models in the testing of skin therapies or wound agents? Do you experience that primary keratinocytes quickly senesce, vary from donor to donor or become prohibitively expensive?
In the meantime, there is pressure on reproducibility, and translational gaps between the bench and the bedside keep growing.
In 2025, the world skincare market is already over USD 115.65 billion, and by 2032, this number is going to reach USD 194.05 billion, making the creation of a reliable keratinocyte model not only necessary but mandatory.
Enter HaCaT cells, an immortalized human keratinocyte line that demonstrates stability, scalability, and physiologically appropriate behavior.
Having their origin in the adult human skin, these freely established immortalized keratinocyte cell lines maintain the ability of differentiation and stratification and are one of the most relevant in vitro systems in terms of physiological significance.
Their stability, scalability, and sensitivity to environmental stimuli enable the researchers to simulate wound healing, barrier function and inflammatory processes with unmatched reproducibility.
Wanted to know more about the role of HaCat cells in skin and wound healing research? Keep reading!
Why HaCaT Cells Matter?
The human skin biology and wound repair can only be studied through reliable cellular models.
Primary keratinocytes, though physiologically relevant, have the drawbacks of donor variability, premature senescence, and poor proliferation, which makes reproducible research difficult.
HaCaT cells, an immortalized human keratinocyte line, are a stable cell type that exhibits the functional characteristics of primary cells, forms stratified layers and reacts in a predictable manner to stimuli.
In order to appreciate their importance, it is vital to learn the constraints of primary keratinocytes first and the reason why scientists have always experienced some difficulty using these cells.
1. Challenge of Primary Keratinocytes
The inherent limitation of primary keratinocytes is that there is variability in donors, they have limited proliferation capacity, and frequent batch-to-batch inconsistency plagues experiments.
Early senescence or barrier phenotype loss is observed in most of the labs after a few passages. This complicates the replication of results or scaling of studies across a number of laboratories.
2. HaCaT as a Stable Alternative
HaCaT is a spontaneously immortalized, aneuploid human keratinocyte cell line that was derived from adult skin. They serve as a reproducible model due to their ability to retain differentiation capacity and form stratified layers.
They are stable in growth and responsive to stimuli, which is why they are good for mechanistic work, screening, and modeling skin physiology.
3. Relevance to Skin, Wound, and Disease Research
HaCaT cells have become popular in proliferation, migration, cytokine response, barrier integrity, and differentiation assays. In wound healing, they are used as surrogate epithelial layers to evaluate scratch closure, migration and growth factor or biomaterial responses.
As an illustration, in a recent study, HaCaT cells underwent exposure to hyaluronic acid formulations and were followed up within a 6-24 h period to study the modulation of wound repair.
In another study, FOSL1 expression in HaCaT has a significant positive effect on migration and proliferation, which validates its involvement in epidermal wound repair through IL-17 signaling.
Therefore, HaCaT offers a physiological relevance and practical robustness, spanning the differences between primary keratinocytes and immortalized, non-skin lines.
Understanding HaCaT Cell Culture Protocols
HaCaT cell culture protocols must be followed carefully when using HaCaT cells. Handling appropriately not only protects reproducibility but also conserves physiological behavior that makes these keratinocytes so useful in skin and wound research.
Every step, from selecting an appropriate medium to avoiding contamination, has a direct impact on the success of all experiments and is an important contributor to a solid HaCaT cell culture protocol.
1. Basal Medium & Supplements
Any HaCaT culture is based on the growth medium.
The most commonly used base is Dulbecco Modified Eagle Medium (DMEM, high glucose) as it offers uniform nutrient support. In order to enhance viability, 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin are usually added to the medium.
In order to achieve specialized results or differentiation endpoints, other variables like nonessential amino acids, L-glutamine, or hydrocortisone can be added- decisions which must be recorded in your standard HaCaT cell culture procedures.
2. Seeding Density & Passage
Cell density and passage strategy are given importance after medium preparation.
To ensure the highest viability, HaCaTs are typically seeded at a density of ~1 × 10⁵ cells/cm² to promote initial recovery after thawing. When doing maintenance, expand cells up to 70-80% confluence before passaging to prevent contact inhibition artifacts.
Though HaCaTs are immortal, phenotypic drift may occur with extreme passage numbers (e.g., >50–60), and hence periodic marker validation assays are advisable and should be included in your documented HaCaT cell culture protocols.
3. Differentiation Induction
In order to examine stratification and the development of barriers, numerous laboratories adopt the Ca²⁺ switch protocol, where the calcium concentration is raised to initiate differentiation marker expression (e.g., involucrin, filaggrin).
In cases of increased physiological relevance, organotypic co-cultures of fibroblasts in 3D matrices re-create in vivo-like epidermal architecture.
4. Cryopreservation & Thawing
Cryopreservation and thawing have to be optimized so that a good cell bank can be maintained. Freeze HaCaTs in 10% DMSO in FBS in a controlled-rate freezer in order to reduce the effects of stress.
The rapid thawing should be followed by the dilution of the thaw into the warm medium, followed by a 24-48 hour recovery period before the experiments to allow restoring viability and consistent behavior.
5. Contamination Control, Authentication & Sourcing
Quality management is fundamental. Silent contaminants are spotted by regular mycoplasma screening, and cell line identity is verified with STR profiling, which is of special concern when obtaining lines between labs or commercial suppliers.
To prevent the cross-lab variability, when acquiring cells from various HaCaT cell suppliers, keep a record of the lot number, passage at receipt, and the authentication report. Lastly, negative and positive controls should be incorporated in the assay to enhance reproducibility.
Adherence to these best practices in their HaCaT cell culture procedures can help researchers ensure stability, reproducibility, and physiological relevance in skin biology, wound healing, and keratinocyte behavior studies.
HaCaT Cells in Dermatology Research
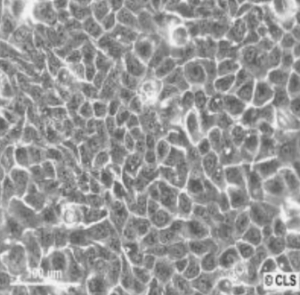
HaCaT cells in dermatology research are one of the most reliable models as they fill in the gap between laboratory experiments and skin biology in the real world. Their diversity enables scientists to examine all aspects of the barrier function and inflammation, as well as wound healing and cosmetic safety, thus being a pillar in the development of skin science.
1. Barrier Function & Skin Biology
HaCaT cells are useful in modelling skin barrier development, UV response, oxidative stress response, cosmetics, and topicals. As an illustration, researchers have been using them to test vitamin D3 metabolism pathways since they express them like the human epidermis.
They are also useful in the research of extracellular vesicles (EVs): HaCaT-derived EVs are demonstrated to induce migration and proliferation in keratinocytes and fibroblasts, and stimulate wound healing reactions in vitro.
2. Inflammation, Signaling, and Modulators
Since HaCaT cells are able to express various cytokine receptors and react to inflammatory signals (e.g., TNF-α, IL-1β), they can be used to investigate pathways relevant to psoriasis, dermatitis, atopic skin, and others.
3. Cosmetic and Safety Testing
HaCaT provides a reliable cytotoxicity, irritation, absorption, or barrier disruption assay model in the development of skin products. Their strength permits screening of several candidate molecules under the same conditions.
4. Wound Healing & Scar Models
To study cross-talk and re-epithelialization processes, in wound healing models HaCaT, researchers tend to do scratch assays, transwell migration, and co-culture with fibroblasts or endothelial cells.
For instance:
- In a study conducted on concentrated growth factor (CGF), the researchers treated HaCaT cells and observed an increase in migratory and proliferative behavior of the cells, which validates that CGF promotes wound reepithelialization.
- TGF-β induction in HaCaT in chronic wound models caused an ER stress response and senescence, which was alleviated by natural extracts and stimulated healing.
Additionally, scientists typically include HaCaT in the upper layers of biomaterials or hydrogels used to treat wounds to recapitulate the epidermal side. For example, in the case of an engineered scaffold of burn wounds, HaCaT cells were encapsulated to balance the features of hydration and barriers.
Considering the demand for new skin therapies around the world, HaCaT-based assays present a high-throughput and reproducible platform to measure candidate molecules, biomaterials, nanocarriers, or gene modifiers.
Design and Interpretation of Wound Healing Models Using HaCaT
In the formulation of wound healing models with HaCaT cells, planning is the key aspect that can distinguish between useful data gathered to scale, and a loud, inconsistent data set.
Various models bring to the fore varying facets of keratinocyte biology, and by choosing the appropriate model, researchers can more effectively describe the complexity of skin repair.
1. Scratch / Wound Closure Assay
A scratch assay is one of the most commonly used techniques, and in it, a linear wound is drawn on a confluent HaCaT monolayer, typically using a pipette tip.
Scholars subsequently track down the closure at time intervals of 0, 6, 12, or 24 hours, using imaging software to quantify recovery. Interpretation, however, needs to be cautious: it is necessary to incorporate reasonable controls–such as a vehicle, and a positive control like epidermal growth factor (EGF) to prove responses.
Moreover, the confirmation of closure as opposed to proliferation through the use of mitotic inhibitors is essential. Medium conditions are important, even as unnecessary serum starvation may decrease viability and skew results.
2. Transwell / Boyden Chamber Migration
Although the scratch assay simulates physical damage, the Transwell migration assay solely concentrates on chemotaxis. In this case, HaCaT cells are seeded in the upper chamber while a chemoattractant is in the lower chamber.
Migrated cells are counted and stained after a predetermined period of time. This design allows the researcher to isolate directed migration and the mechanical disruption due to scratching and provides a less complicated view of how keratinocytes react to particular growth factors or signaling molecules.
3. Co-culture with Fibroblasts or Endothelial Cells
HaCaTs are frequently co-cultured with fibroblasts or endothelial cells to more closely mimic in vivo conditions.
The basal layer in fibroblasts synthesizes extracellular matrix signals and growth factors, which directly influence HaCaT behavior, while endothelial cells provide another dimension of cross-talk.
These systems enable the researcher to investigate paracrine communication and examine how the skin microenvironment causes keratinocyte migration, proliferation and differentiation.
4. 3D / Organotypic Models
To achieve even greater fidelity, scientists employ 3D organotypic cultures.
Under this system, the dermal fibroblasts are seeded into a collagen gel and covered with HaCaTs, which are subsequently lifted to the air-liquid interface. In this 3D architecture, wounding can be induced, and re-epithelialization is monitored over time.
This method more realistically reflects skin biology in vivo, especially the study of barrier properties, stratification and morphogenesis.
5. Mechanistic Readouts
Lastly, no wound healing model is comprehensive without mechanistic readouts to describe the observed effects.
Molecular methods such as qRT-PCR and western blotting are capable of monitoring the expression of essential markers, including Ki67 or PCNA for proliferation, MMPs and integrins for migration and keratins or involucrin for differentiation.
Imaging techniques such as immunofluorescence, confocal microscopy, or live-cell time-lapse provide spatial and temporal insights into cytoskeletal dynamics and junction protein localization.
Researchers can also apply inhibitors to pathways like MAPK or PI3K to tease apart the molecular mechanisms driving HaCaT responses.
By combining these complementary approaches, HaCaT-based wound healing models allow scientists to capture the full spectrum of keratinocyte behavior—from migration and proliferation to inflammation and barrier regeneration.
The result is a set of versatile, reproducible tools that bridge the gap between simplified in vitro assays and the complexity of human skin repair.
Strengths, Limitations & Best Practices
Like any research model, HaCaT cells come with clear strengths as well as important limitations, and recognizing both is key to designing reliable, insightful experiments.
Strengths
- Reproducibility: Unlike donor-derived cells, HaCaT cells provide stable, consistent behavior.
- Scalability: Efficient expansion enables screening of many conditions.
- Physiological Relevance: Maintain many keratinocyte traits, stratification potential, and responsiveness.
- Versatility: Suitable for 2D, 3D, co-culture, and mechanistic studies.
Limitations
- Immortalization and Aneuploidy: Their transformed nature may diverge from normal keratinocyte physiology.
- No Full Skin Architecture: They lack dermal, vascular, and immune components unless co-integrated.
- Drift Over Passage: At very high passages, phenotypic drift can occur.
- Absence of Melanocytes or Other Skin Subtypes: Alone, they cannot model pigmentation or multicellular skin complexity.
Mitigation & Best Practices
- Restrict passage number; always log passage.
- Include primary keratinocyte or in vivo controls when possible.
- Use co-culture or 3D organotypic models to simulate the microenvironment.
- Validate newly thawed stocks before large experiments.
By understanding both the power and caveats of HaCaT, you can design experiments that leverage its strengths while mitigating its weaknesses.
HaCaT Cells Driving Research Forward
HaCaT cells stand at the intersection of practicality and physiological relevance, offering researchers a powerful tool for dermatology, wound healing, and skin therapeutics research.
By following rigorous HaCaT cell culture protocols, selecting reliable HaCaT cell suppliers, and thoughtfully designing wound healing models with HaCaT cells, you can reduce experimental variability and accelerate discovery.
As the global skin-health market expands and demand for translational insight intensifies, HaCaT cells will continue to anchor innovations in keratinocyte biology, biomaterials, cosmeceuticals, and regenerative medicine.